Protein Evolution & Delivery
The laboratory evolution and in vivo delivery of proteins with therapeutic or biotechnological potential
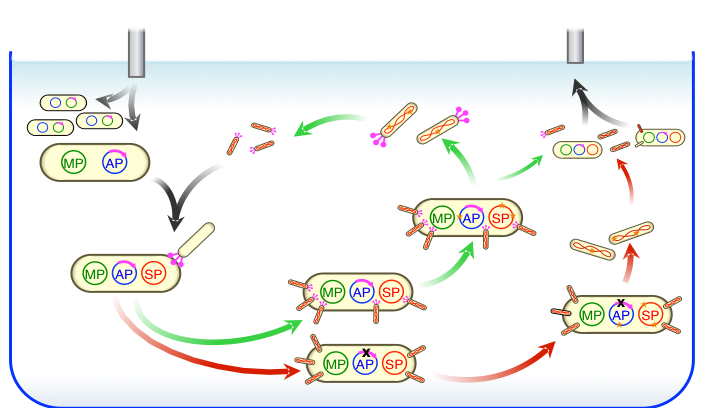
During PACE, host E. coli cells flow continuously into a fixed-volume vessel (a lagoon) containing a population of filamentous bacteriophages that encode a library of evolving proteins. The lagoon is continuously drained to a waste container after passing through an in-line luminescence monitor that measures expression from a gene III–luciferase cassette on the AP. Dilution occurs faster than cell division but slower than phage replication. Each phage carries a protein-encoding gene to be evolved instead of a phage gene (gene III) that is required for infection. Phage encoding active library members trigger expression of gene III on the AP in proportion to the desired activity and consequently produce infectious progeny. Phage encoding less active library members produce fewer infectious progeny and are lost by dilution. From: Nat. Chem. Biol. 10, 216-222 (2014). PDF
PROTEIN EVOLUTION Biological evolution has efficiently solved many challenging molecular problems. By harnessing the power of biological evolution, researchers have started to address problems of their own choosing, rather than of nature’s choosing. The Liu group has developed a method that enables proteins to evolve continuously in the laboratory known as phage-assisted continuous evolution (PACE), increasing the speed of laboratory evolution by more than 100-fold over other methods. We applied PACE to evolve more than a dozen diverse classes of proteins including proteases, genome editing proteins, polymerases, antibodies, biosynthetic enzymes, and insecticidal proteins. PACE is now used both in academic and industry settings to evolve proteins with dramatically altered activities and specificities, and to reveal new basic scientific insights into the nature of biological evolution.
Protein Evolution
Video Description: David Liu describes the development and first applications of phage assisted continuous evolution (PACE), a method that enables proteins to evolve continuously in the laboratory for the first time, accelerating the speed of laboratory evolution ~100 fold. PACE has been used to rapidly evolve a wide variety of proteins with the potential to serve as novel therapeutic agents, and to study the reproducibility and path dependence of evolution over thousands of generations in a practical time frame.
Featured Publications: Protein Evolution
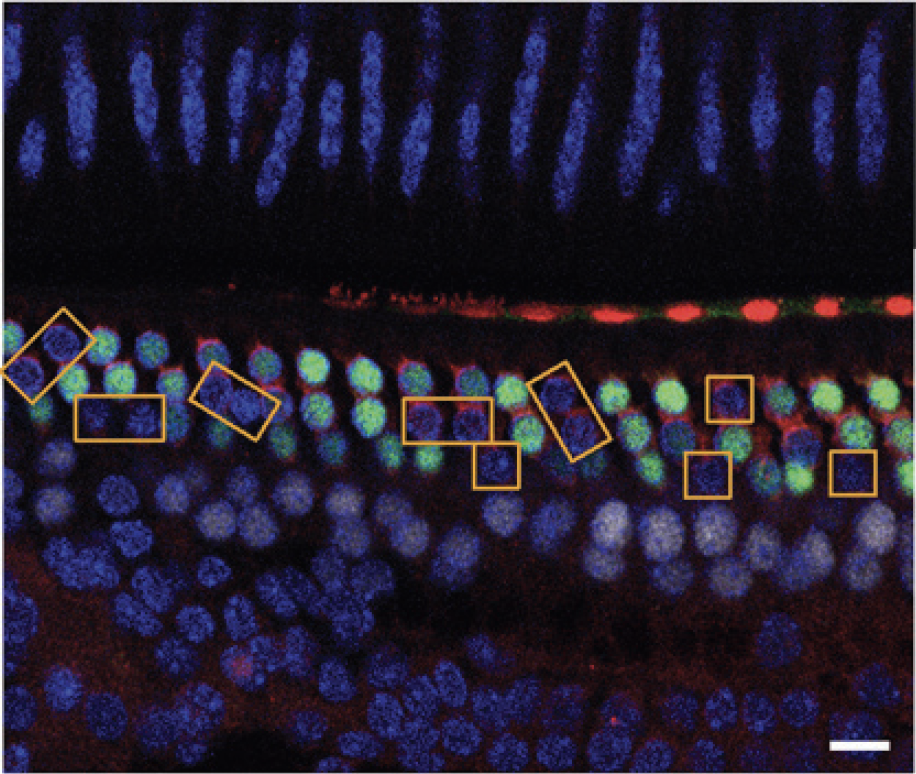
In vivo delivery of Cre recombinase and Cas9:sgRNA complexes to hair cells in the mouse inner ear. Yellow boxes highlight hair cells that have lost GFP expression. Scale bar (white), 10 mm. Credit: Zuris, J. A.; Thompson, D. B.; Shu, Y.; Guilinger, J. P.; Bessen, J. L.; Hu, J. H.; Maeder, M. L.; Joung, J. K.; Chen, Z.-Y.; Liu, D. R. Nat. Biotechnol. 33, 73-80 (2015)
PROTEIN DELIVERY Maximizing the therapeutic relevance of proteins requires the ability to effectively deliver them into mammalian cells, of the appropriate type in vivo. We discovered that